,
by Shana Spindler
For folks with polycythemia vera, therapy with the drug rusfertide seems to considerably scale back their reliance on blood attracts to keep away from severe well being issues. That’s in accordance with outcomes of the first-ever medical trial to check a drug like rusfertide—which blocks the manufacturing of iron-rich blood cells—in folks with this continual blood most cancers.
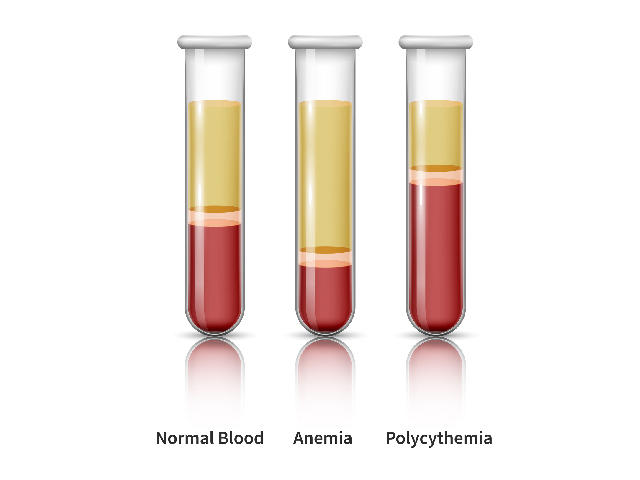
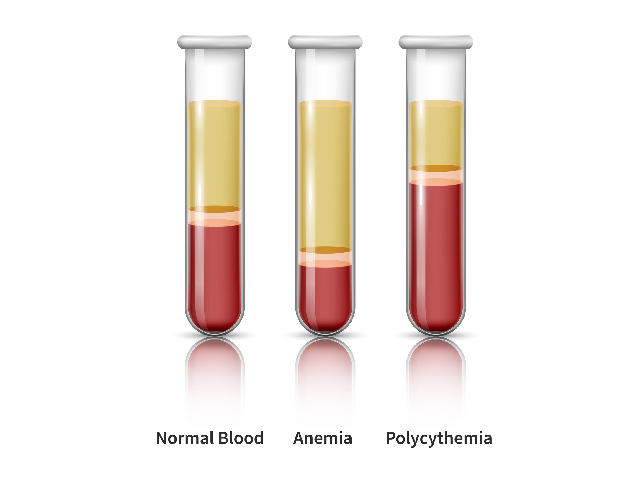
In folks with polycythemia vera, the bone marrow makes too many purple blood cells. This overproduction of cells thickens the blood and, if left untreated, will increase the chance for blood clots, coronary heart assault, and stroke.
For many years, the principle therapy for polycythemia vera has been common blood attracts, or phlebotomy, to take away extra purple blood cells. However this rudimentary therapy has downsides, together with debilitating fatigue and mind fog. The necessity to make common journeys to a physician’s workplace for the process can also be time consuming and impacts folks’s high quality of life.
“Principally, in a manner, we nonetheless deal with sufferers with polycythemia vera by bloodletting,” stated Marina Kremyanskaya, M.D., Ph.D., of the Icahn Faculty of Drugs at Mount Sinai in New York Metropolis, who led the research.
So Dr. Kremyanskaya started finding out therapies that particularly suppress purple blood cell formation—calling it a “chemical phlebotomy.” A number of of those medicine, referred to as hepcidin mimetics, are at the moment being studied in medical trials. Rusfertide is the furthest alongside in testing.
The trial, known as REVIVE, included 70 folks with polycythemia vera who required frequent phlebotomies to maintain their purple blood cell numbers low. Including rusfertide to their ongoing therapy of phlebotomy had a significant influence, Dr. Kremyanskaya stated.
For many members who obtained rusfertide, “[their] want for phlebotomy was considerably lowered or eradicated,” she stated, dropping from a median of 9 phlebotomies per yr earlier than the trial to lower than one phlebotomy per yr after beginning rusfertide.
Outcomes of the trial have been printed February 22 within the New England Journal of Drugs.
“Folks with polycythemia vera nonetheless wish to dwell a traditional life … and the issue is that plenty of sufferers are [constantly] very drained once they obtain phlebotomy” time and again, stated Idoroenyi Amanam, M.D., of the Metropolis of Hope, who treats folks with blood cancers like polycythemia vera however was not concerned within the trial.
The findings from this trial are “thrilling,” he stated. However rusfertide is just not but permitted by the Meals and Drug Administration (FDA) to deal with polycythemia vera. And for now, he continued, rusfertide is barely out there for these collaborating in a medical trial.
Remedy choices for polycythemia vera
The proportion of blood quantity that’s composed of purple blood cells is named the hematocrit. For these dwelling with polycythemia vera, the purpose of therapy is to take care of a hematocrit beneath 45%.
To make sure that they keep beneath that degree, folks with polycythemia vera repeatedly go to their physician to verify their hematocrit. If it’s too excessive, they then bear therapeutic phlebotomy—a course of akin to blood donation—during which a big needle is inserted right into a vein to take away blood from the physique. The method takes anyplace from 10 to half-hour, in accordance with Dr. Kremyanskaya, however the workplace go to in complete can stretch for much longer and intrude with folks planning their lives.
Folks with polycythemia vera who usually tend to have issues like blood clots might obtain further therapies to scale back their purple blood cell counts. However these therapies even have downsides, together with low blood counts and infections. The underside line, Dr. Kremyanskaya stated, is that, past phlebotomy, “there are literally only a few therapy choices.”
Including rusfertide improves blood counts and signs
Rusfertide works by imitating a pure hormone produced by the liver known as hepcidin, which controls iron metabolism—that’s, how a lot iron is absorbed from meals by the intestines and the way a lot iron is launched by iron-storing cells into the bloodstream.
Iron is necessary for lots of mobile processes, however particularly for purple blood cell manufacturing, Dr. Kremyanskaya defined.
Remedy with rusfertide basically methods the physique into behaving as if it has greater hepcidin ranges, which causes iron ranges to drop within the bloodstream, she continued. And with out iron, purple blood cell manufacturing stops.
Certainly, findings from laboratory and animal mannequin research urged that rising hepcidin exercise within the physique might doubtlessly restrict purple blood cell manufacturing. Researchers have been testing numerous therapies that make the most of this discovering, together with the hepcidin imitator rusfertide.
The REVIVE research—funded by Protagonist Therapeutics, the producer of rusfertide—is split into a number of elements. The findings lately printed within the New England Journal of Drugs are from the primary two elements of the research.
Within the first a part of the research, which spanned 7 months, all members obtained an injection of rusfertide as soon as per week and continued their ongoing remedy (phlebotomy alone or together with different remedy to scale back blood counts) as wanted. Within the second a part of the research, which lasted an extra 3 months, 59 members have been randomly assigned to stay on rusfertide or swap to a placebo. The purpose was to evaluate what would occur when rusfertide was withdrawn.
Earlier than starting therapy with rusfertide, the imply most hematocrit in members was 50%. That degree fell to 44.5% as soon as rusfertide was added to their therapy. In consequence, virtually not one of the members wanted a phlebotomy throughout the first a part of the research.
As well as, members reported lowered severity of their signs, corresponding to fatigue, evening sweats, issues concentrating, and itching.
Partly two of the trial, 60% of those that stayed on rusfertide however solely 17% of those that have been switched to placebo had what the trial leaders outlined as a response: they maintained a hematocrit beneath 45% and didn’t want a phlebotomy.
The commonest facet impact of rusfertide was a response on the injection website, Dr. Kremyanskaya stated. For individuals who had a nasty response, the injection website might be purple, painful, and itchy. However, she famous, these bothersome reactions lessened because the members remained on the drug.
VERIFYing the profit
Rusfertide seems to assist folks with polycythemia vera preserve wholesome hematocrit ranges with out the necessity for phlebotomy for greater than 2 years, in accordance with findings from the third and last a part of the REVIVE trial, introduced on the American Society of Hematology Annual Assembly in December 2023.
As encouraging because the REVIVE findings are for the lowered want for phlebotomy, the symptom knowledge got here from individuals who weren’t randomized within the first a part of the trial, “and so we’ve got to take it with a grain of salt and see what the randomized knowledge exhibits,” Dr. Kremyanskaya stated.
Extra definitive findings will come from a bigger part 3 trial, known as VERIFY, that’s now underway. Not like the REVIVE research, during which all members obtained the drug up entrance, the VERIFY trial will randomly assign members to obtain rusfertide or placebo from the start of the trial.
Watching for added well being issues
One necessary query to reply is that if rusfertide reduces the chance of coronary heart assault and stroke in folks with polycythemia vera, Dr. Amanam famous. “The time-frame [in the trial] was very brief for follow-up, so we don’t have that info but,” he stated.
One other necessary difficulty to observe is second cancers. Folks with polycythemia vera have an elevated danger of each pores and skin most cancers and acute myeloid leukemia (AML), though it’s unclear why. Fueling the priority, some present polycythemia vera therapies—such because the chemotherapy hydroxyurea—are additionally identified to extend pores and skin most cancers danger.
As a part of the REVIVE trial, all members obtained pores and skin examinations by a dermatologist earlier than they started therapy and repeatedly throughout the research.
Whether or not or not rusfertide will increase the chance of pores and skin most cancers is unknown, “however we should be vigilant about monitoring,” Dr. Kremyanskaya emphasised.
As well as, Dr. Amanam stated it is going to even be necessary to see if rusfertide therapy helps management the development from polycythemia vera to AML.
However he’s hopeful that rusfertide might finally provide a brand new choice for folks with polycythemia vera to recapture a part of their lives. “Folks wish to know, ‘Will this assist me really feel higher … and can I be capable to dwell my life in a manner that I prefer to dwell it?’” he stated.
Protagonist Therapeutics stated it plans to hunt FDA approval for rusfertide for treating polycythemia vera in late 2025.
With a number of medical trials testing different hepcidin mimetics already ongoing, there’s a sense amongst researchers that managing polycythemia vera could also be reaching a turning level, Dr. Kremyanskaya stated. “This can be a new space of analysis in polycythemia vera that hopefully will result in one other [treatment option] for sufferers.”