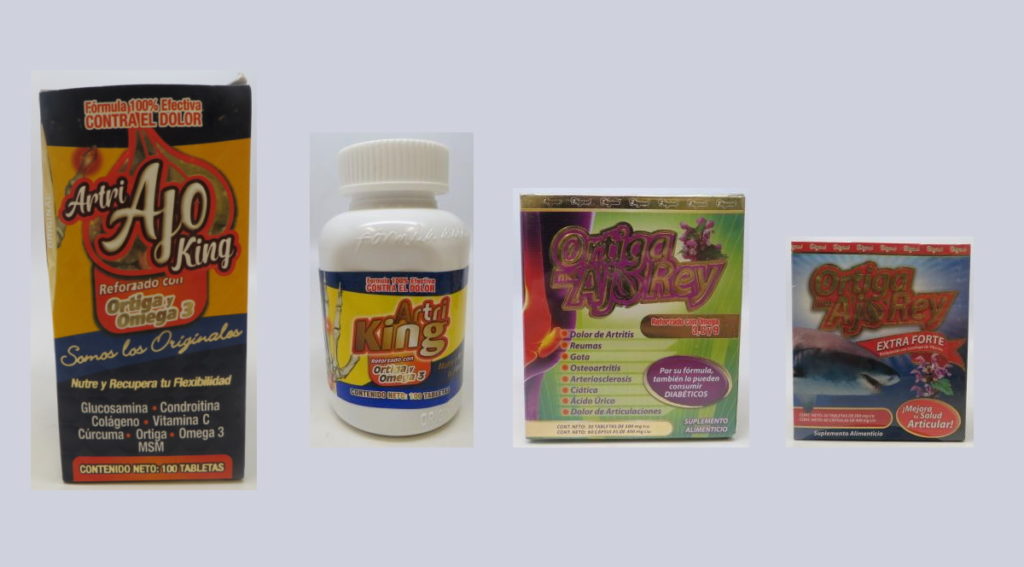
Merchandise marketed with the names “Artri” or “Ortiga” might comprise hidden energetic drug components that will pose a well being danger to sufferers, based on the Meals and Drug Administration (FDA).
The Company has decided that Artri Ajo King, Artri King, Ortiga Mas Ajo Rey, and Ortigo Mas Ajo Rey Further Forte all comprise undeclared drug components that will fluctuate in efficiency from product to product or lot to lot. Laboratory analyses revealed that these merchandise comprise energetic drug components, together with dexamethasone (a corticosteroid), diclofenac sodium (a nonsteroidal anti-inflammatory drug), and methocarbamol (a muscle relaxant).

The merchandise, that are offered on-line and in retail shops, are being promoted as therapies for arthritis, muscle ache, osteoporosis, bone most cancers and different situations. Antagonistic occasions, together with liver toxicity and loss of life, have already been reported with the usage of Artri King merchandise.
The FDA recommends that well being care suppliers consider sufferers who’ve used Artri and Ortiga merchandise for drug and illness interactions. Antagonistic occasions associated to those merchandise must be reported to the FDA’s MedWatch program.
Investigation into the distribution of Artri and Ortiga merchandise within the US is ongoing.
Reference
FDA warns shoppers to not buy or use Artri and Ortiga merchandise, which can comprise hidden drug components. Information launch. April 20, 2022. https://www.fda.gov/medication/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-artri-and-ortiga-products-which-may-contain-hidden-drug
This text initially appeared on MPR